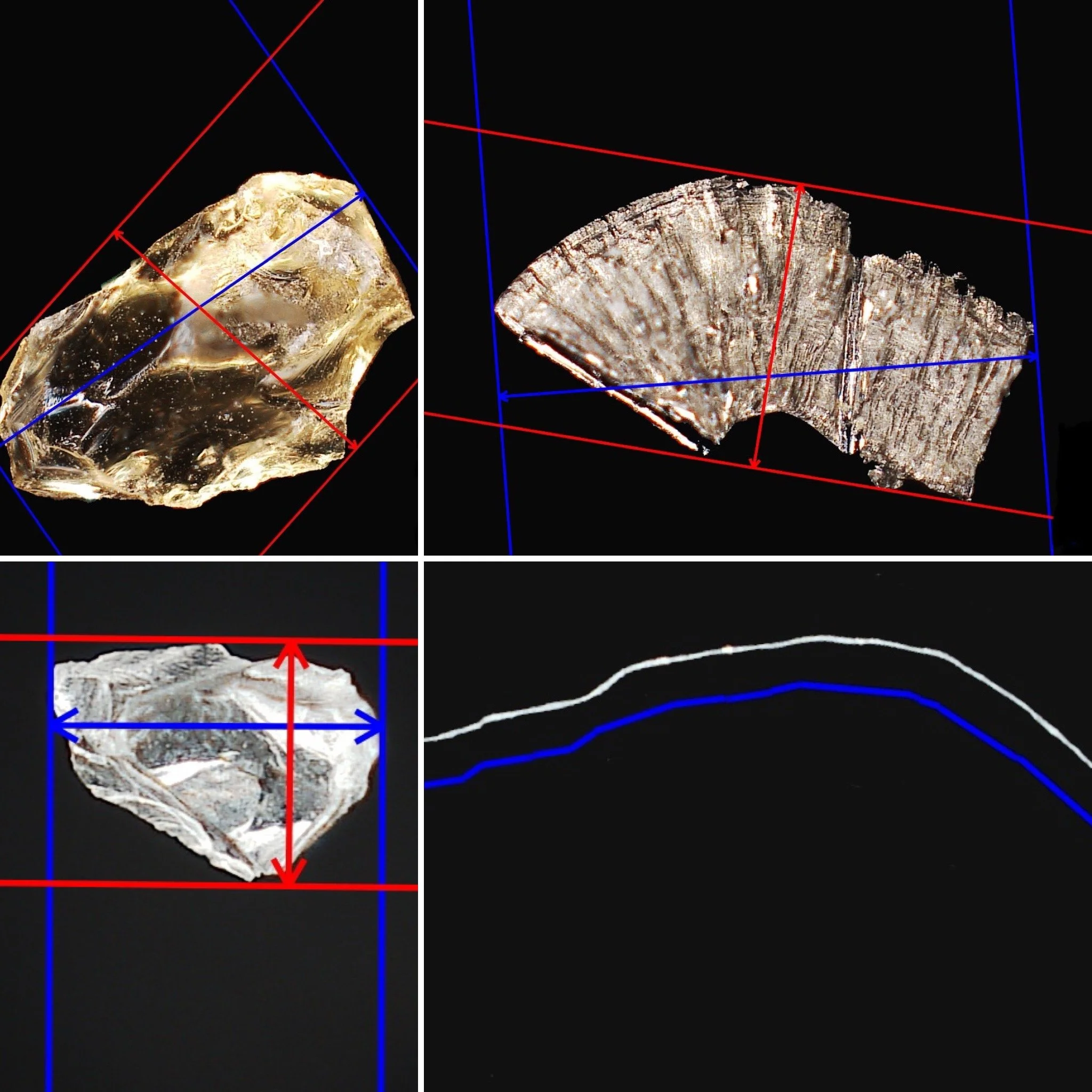
DEFECT SAMPLES
Pharmaceutical companies are challenged by the need to ensure the highest quality in parenteral products, therefore requiring manual, semi-automatic, or automatic inspection to detect and reject products with contaminants or defects.
test standards
why use test standards?
Processes and machinery must be validated to comply with FDA regulations and ensure they perform as intended. Test Standards (aka control sets) are used to measure the efficiency of the inspection process, whether manual, semi-automatic, or automatic. They can also be used to perform routine challenges to verify that the process maintains its validated state or to perform Knapp statistical studies. They can also be used to train inspectors for a manual inspection process.
what we do
Our validation professionals have experience with inspection machinery, having validated over 165 automatic inspection machines worldwide and the development of inspection recipes. We know how to best fabricate the test set to perform at its best on your machine since we have experience with most brands of inspection machines. We prepare the sets in ISO Class 5 (209E Class 100) laminar flow hoods, located in an ISO Class 7 cleanroom (ISO 14644-1; Fed Std 209E Class 10,000).
Materials used
We use particulate matter made out of our customers' containers to represent the type of particulate that could be encountered in the production area. Particles are measured and classified, then seeded into the containers with Water for Injection, or product solution. Cosmetic defects are created manually, measured and identified for size and location. We work with a variety of vials, ampoules, cartridges, and syringes in different sizes.
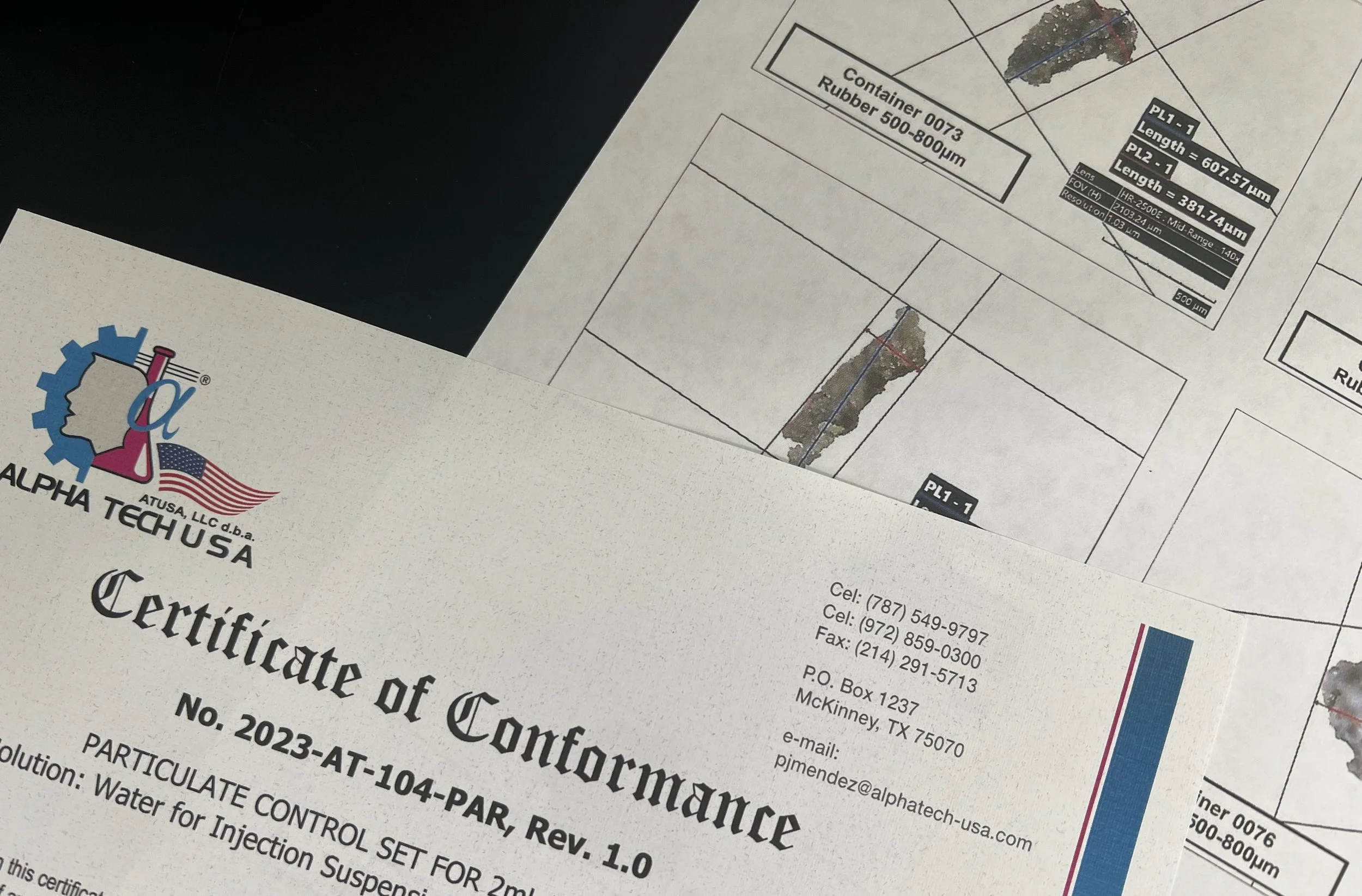
certificate of conformance
All test standard sets are delivered with a Certificate of Conformance. We can produce a photographic record with individual descriptions and measurements for each particulate in the set.